how to make an ester What are the uses of esters?
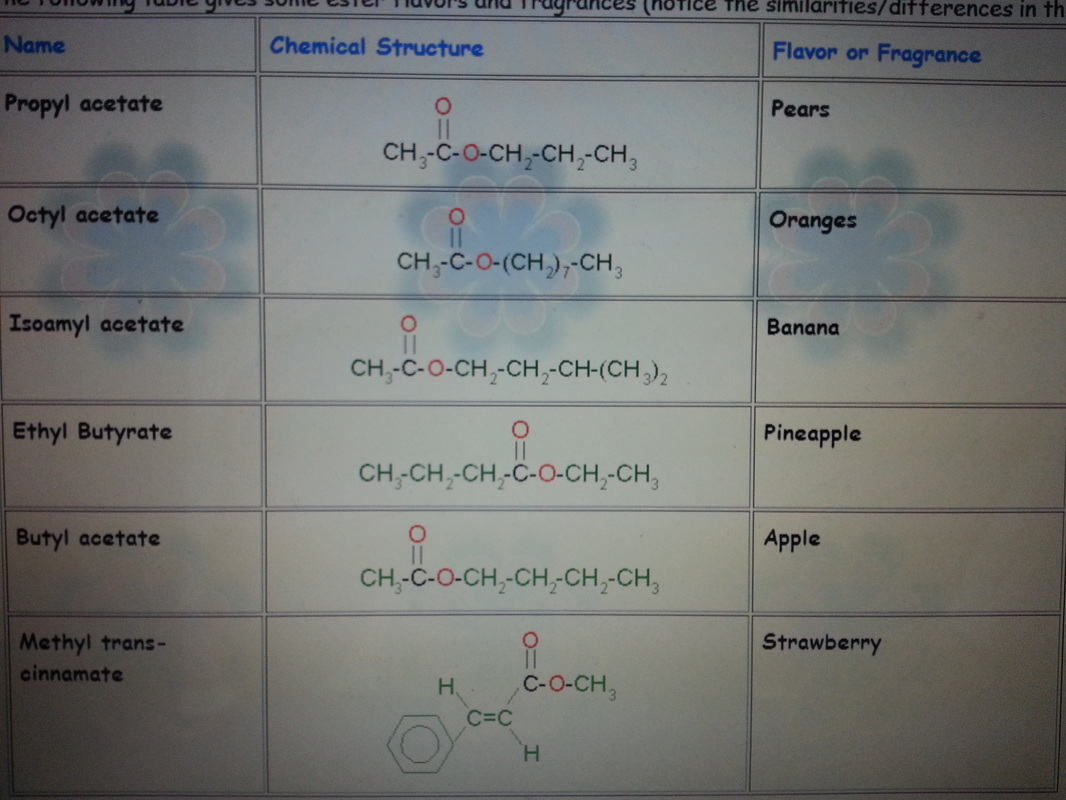
Esters are important organic compounds that are widely used in various industries and everyday life. They have a distinct fruity or floral scent and are responsible for the aroma of many fruits, flowers, and perfumes. In addition to their pleasant fragrance, esters also play a crucial role in the synthesis of numerous pharmaceuticals, flavors, and fragrances.
Boiling Point of Ester and Carboxylic Acid
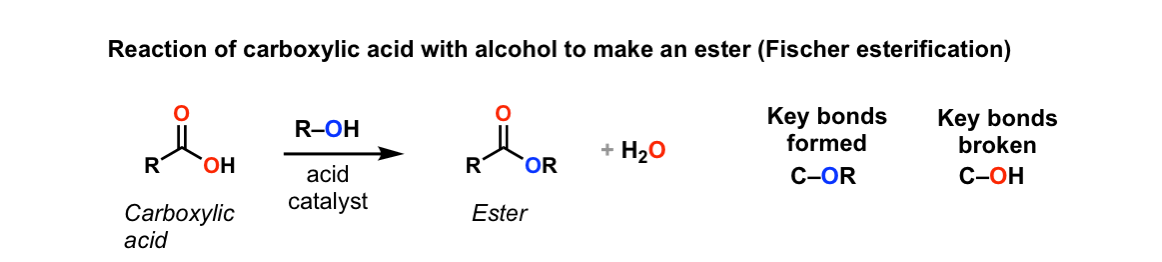 One of the key aspects commonly discussed when it comes to esters is their boiling point. Esters generally have lower boiling points compared to carboxylic acids. This difference arises due to the presence of polar functional groups, such as the carboxyl group (-COOH), in carboxylic acids. These polar groups allow for stronger intermolecular forces, such as hydrogen bonding, resulting in higher boiling points.
One of the key aspects commonly discussed when it comes to esters is their boiling point. Esters generally have lower boiling points compared to carboxylic acids. This difference arises due to the presence of polar functional groups, such as the carboxyl group (-COOH), in carboxylic acids. These polar groups allow for stronger intermolecular forces, such as hydrogen bonding, resulting in higher boiling points.

Conversion of Carboxylic Acids to Esters
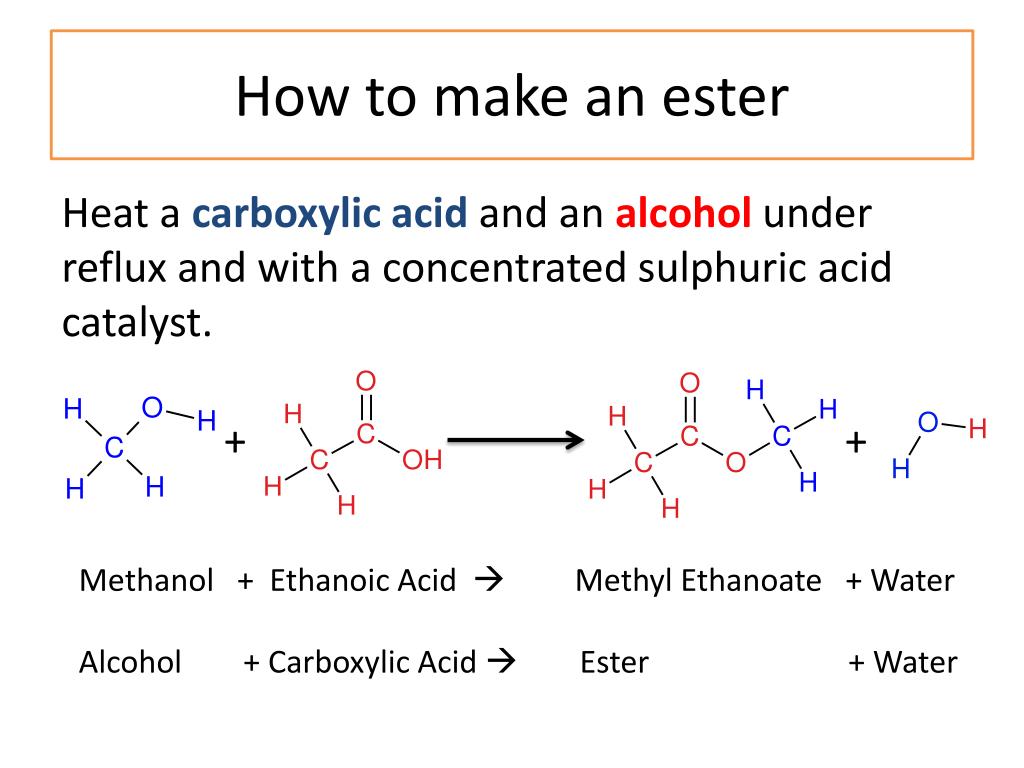
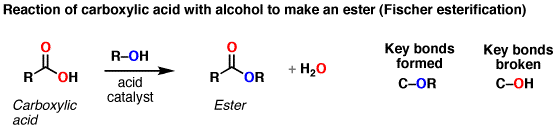
The conversion of carboxylic acids to esters is a fundamental process in organic chemistry. One of the most commonly used methods for this conversion is the Fischer esterification. It involves the reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst, typically sulfuric acid. The reaction leads to the formation of an ester and water as a byproduct.
During the Fischer esterification, the carboxylic acid molecule undergoes nucleophilic addition with the alcohol molecule. The alcohol replaces the carboxylic acid’s hydroxyl group (-OH) to form an ester functional group (-COO-). The acid catalyst helps in the protonation of the carbonyl oxygen and facilitates the reaction.
The conversion of carboxylic acids to esters is of great significance in industries such as pharmaceuticals and flavors. It allows for the synthesis of various ester derivatives that possess different scents and flavors. For example, the conversion of acetic acid with ethanol leads to the formation of ethyl acetate, which is commonly used as a solvent and flavoring agent in various food products.
Esters are not only valuable in the fragrance and flavor industries but also find applications in the synthesis of pharmaceutical compounds. Many ester derivatives are utilized as prodrugs, which are inactive compounds that undergo conversion to the active drug form in the body. This method allows for controlled drug release and enhanced therapeutic efficacy.
In conclusion, esters are versatile compounds with a wide range of applications. Their boiling points, lower than those of carboxylic acids, make them suitable for various uses. The conversion of carboxylic acids to esters, especially through Fischer esterification, is a vital process in organic chemistry that enables the synthesis of valuable fragrance, flavor, and pharmaceutical compounds.
If you are searching about Boiling Point of Ester and Carboxylic Acid - ReynartCruz you’ve visit to the right web. We have 5 Pictures about Boiling Point of Ester and Carboxylic Acid - ReynartCruz like Conversion of carboxylic acids to esters using acid and alcohols, what are the uses of esters? - ORGANIC CHEMISTRY IN OUR DAILY LIFE and also Conversion of carboxylic acids to esters using acid and alcohols. Read more:
Boiling Point Of Ester And Carboxylic Acid - ReynartCruz
 reynartcruz.blogspot.comPPT - Esters PowerPoint Presentation, Free Download - ID:1936696
reynartcruz.blogspot.comPPT - Esters PowerPoint Presentation, Free Download - ID:1936696
 www.slideserve.comWhat Are The Uses Of Esters? - ORGANIC CHEMISTRY IN OUR DAILY LIFE
www.slideserve.comWhat Are The Uses Of Esters? - ORGANIC CHEMISTRY IN OUR DAILY LIFE
 gen2chemistassignment.weebly.comesters uses ester life organic chemistry daily flavours some table labels foods fragrances artificial
gen2chemistassignment.weebly.comesters uses ester life organic chemistry daily flavours some table labels foods fragrances artificial
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols
 www.masterorganicchemistry.comesterification carboxylic fischer ester alcohol acids esters alcohols catalyst along formed
www.masterorganicchemistry.comesterification carboxylic fischer ester alcohol acids esters alcohols catalyst along formed
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols
 www.masterorganicchemistry.comesterification fischer acid carboxylic reaction acids esters alcohols conversion alcohol using water ester catalyst chemistry organic formed equilibrium when treated
www.masterorganicchemistry.comesterification fischer acid carboxylic reaction acids esters alcohols conversion alcohol using water ester catalyst chemistry organic formed equilibrium when treated
Esterification carboxylic fischer ester alcohol acids esters alcohols catalyst along formed. What are the uses of esters?. Conversion of carboxylic acids to esters using acid and alcohols